INTRODUCTION
The concept of Three electron bond was introduced by Pauling (1931) who
described that odd electron are used to form them. Presence of odd
electron in paramagnetic species like O2, He+, CO, NO, NO 2, etc show relevance of three electron bond. The mentioned
molecules contain a paired electron on one atom and an unpaired
electron on another and possess equivalent energy difference [1].
It is combined effect of three electrons with relative spin resulting
in the formation of unique type of bond.These play a vital role in bond
formation and bond cleavage as well as exists as intermediate in
various chemical reactions [2].
The Idea of Three Electron Bond was developed by Heitler and London.
They proposed that an electron bond pair is formed by the interaction
of unpaired electrons in two atoms. It is observed that a stable
molecule is formed when two sharing atoms possess an unpaired electron
[3].
The system consists of a single electron belonging to one nucleus and a
pair of electron belonging to another nucleus refers to the interchange
of three electrons. Here we consider the example of Hydrogen atom
having single electron and a paired electron containing specie Helium
atom.The normal Helium nucleus (He) and Hydrogen nucleus (H) have no
affinity to molecule formation. However, if two nuclei have identical
energy an additional degenerate orbital of the configuration He: .H is formed [4].
|
 H.+ :He
H.+ :He
|
H:.He+
|
(i)
|
|
 H.: He -
H.: He -
|
H: .He
|
(ii)
|
|
 H.: He
H.: He
|
H:-.He+ (iii)
|
Such system refers to two center- three (2c-3e) electron bonding. Such
Lewis structures are resonance stabilized and show charge transfer.
Energy difference must be small to acquire stabilization energy [5, 6].
The Energy difference for the neutral species (iii) shown above
involves least Energy difference between Ionization potential of He and
Electron Affinity of H. There is a distribution of three electrons
between two overlapping atomic orbital [7].
Thus Postulates of three electron bond theory include presence of three
electrons with oppositely oriented spins, consists of atom that has
completed its octet configuration, electron spin adjustment is done in
such a way that the attraction is minimum for bonding [8].
It is mostly observed in radical cation with an interaction of an
unpaired p-orbital. The net energy of the system is calculated as half
the strength of two electron bond system. The Electronegative
difference between the sharing atom must not exceed 0.5.Such bond is
formed when an unpaired electron of an atom combine to the lone pair of
electron of another atom.
Resonance Stabilization
Bond Energy depends on interchanging energy of the two shared pair of
electrons or resonance or the electrostatic force [9]. While drawing
resonance structure we take care that electrons move to adjacent
position neighboring atom or group to form a pi bond and that the net
charge of all resonating structure must be same.
Strongest Three electron bond occur in two identical fragment mostly
and heteroatom from first and second period (like N:.N, O:.O, F:.F,
P:.P, S:.S, Cl:.Cl) rare gases (like He:. He, Ne:.Ne, Ar:. Ar).Various
other molecule like NO, CO2 show three electron bond of
which NO is most stable of the odd electron molecule [10].
Stability of various molecules is explained by the formation of three
electron bond.
The Energy difference between the two resonating structure are used to
determine the stabilization energy [11]. Resonance between several
electronic structures proposed by Lewis is used to determine the bond
distance between two atoms [12]. The two resonant structure
of NO molecule (I & II) proposed by Pauling is noted herewith (Fig.
1).

Fig. 1.
Resonant structure of NO molecule
Other paramagnetic species like He, Ar, O2, NO2,
NO etc. are shown diagrammatically with three dots representing the
three electron bond. The three electron bond prototype having
degenerate levels is seen in di-positive Helium ion [12]. Various
evidence show that a neutral helium molecule is formed by one excited
helium atom containing an unpaired 1s electron and one normal helium
atom that forms Helium band [13]. The same happens for noble gases like
Helium and Argon(Fig. 2) and gasses like nitric oxide and oxygen (Fig.
3).

Fig. 2.
Three dot representation of Three Electron Bond
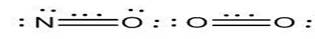
Fig. 3.
Three dot representation of electronegative atom Nitrogen & Oxygen
Various electronegative atoms like Nitrogen and Oxygen have slight EN
difference and same effective nuclear charge. Resonance between the
structures shown lead to a double and a three electron bond [14].
Stabilization of Three Electron Bond
Stability of nucleus showing three electron bonding depends on
resonance exhibited by two Lewis structures which is related to
transfer of charge. It is observed that significant resonance energy is
required for stability of two resonating Lewis Structure [15].
According to Clark, three electron bond energy of ion show an
exponential decrease with the difference in Ionization potential and
the Electron affinity of the Lewis structures. He carried out
systematic calculation on several radical cations involving three
electron bond between various atoms of first and second rows of a
periodic table, substituting hydrogen atom [16].
Three electron bond is however a new concept that involves three
electrons and are distributed among two overlapping atomic orbital. The
hypothesis of three electron bond can be explained by Valence bond
theory and Molecular Orbital Theory. Valence bond theory explains
overlapping of two half filled orbital and when the atomic orbital
contains more than a single unpaired electron, there can be formation
of more than one bond. Molecular orbital Theory represents doubly
occupied Molecular Orbital (MO) and a singly occupied MO of the
molecule He: .H depicting bonding and antibonding
orbital. The distribution of three electron between two overlapping
atomic orbitals is validated by the least energy difference of
Ionization potential and Electron affinity. Thus both Valence Bond
Theory and Molecular Orbital theory divulge into the same conclusion
and the two Lewis structure are mutually related by charge-shift.
REFERENCES
[1] R.D. Harcourt, One-electron and three-electron chemical bonding,
and increased-valence structures, Theo. Comput. Chem. 1999 (2000)
449-480.
[2] S. Zhang, X. Wang, Y. Su, Y. Qiu, Z. Zhang, X. Wang, Isolation and
reversible dimerization of a selenium three-electron s-bond, Nature
Communications, 5 (2014) 4127.
[3] L. Pauling, The nature of the chemical bond. II. The one-electron
bond and the three-electron bond, J. Am Chem. Soc. 53 (1931) 3225-3237.
[4] L. Pauling, The nature of the chemical bond. III. The transition
from one extreme bond type to another, J. Am. Chem. Soc. 54 (1932)
988-1003.
[5] T, Clark. Odd electron sigma bond, J. Am. Chem. Soc. 110 (1988)
1672-1678.
[6] L.O. Brockway, The Three Electron bond in Chlorine Dioxide, Proc.
Natl. Acad. Sci. 19 (1933) 303-307.
[7] B.V. Dmytrovych, Theory of Three-Electron Bond in the Four Works
with Brief Comments, Org. Chem. Curr. Res. 6 (2017) DOI:
10.4172/2161-0401.1000182
[8] L. Pauling, The nature of the chemical bond. II. The one-electron
bond and the three-electron bond, J. Am. Chem. Soc. 53 (1931)
3225-3237.
[9] L. Pauling, The nature of the chemical bond. Application of results
obtained from the quantum mechanics and from a theory of paramagnetic
susceptibility to the structure of molecules’, J. Am. Chem. Soc. 53
(1931) 1367-1400.
[10] I. Fourr´e, B. Silvi, What Can We Learn from Two-Center
Three-Electron Bonding with the Topological Analysis of ELF, Heteroatom
Chem. 18 (2007) 135-160.
[11] T. Clark, Odd electron s bond. J. Am. Chem. Soc. 110 (1988) 1672-
1678.
[12] L. Pauling, The Electronic Structure of the Normal Nitrous Oxide
Molecule. Proc Natl. Acad Sci. 4 (1932) 498-499.
[13] W. Weizel, Z. Physik, The structure of bond spectrum of Helium. J.
Am. Chem. Soc. 59 (1929) 3225-3237.
[14] G.N. Lewis, Valence and the Structure of Atoms and Molecule, J.
Am. Chem. Soc. 128 (1923)
[15] D. Danovich, C. Foroutan-Nejad, P. Charles Hiberty, S.A. Shaik, On
the Nature of the Three-Electron Bond, J Phys. Chem. 38 (2018).
[16] K. Bobrowski, J. Holcman. Formation and stability of
intramolecular three-electron SN, SS, and SO bonds in
one-electron-oxidized simple methionine peptides. Pulse radiolysis. J.
Phys. Chem.9