Formulation and characterization of an analgesic ointment indicated for
pediatric use
Uttam Kumar Mandal*; Anis Arabi Hashmi; Bappaditya
Chatterjee
IKOP Sdn. Bhd., Kulliyyah of Pharmacy, International
Islamic University Malaysia (IIUM), Jalan Sultan Ahmad Shah, Bandar
Indera Mahkota, 25200, Kuantan, Pahang, Malaysia.
*
Corresponding author:
Dr. Uttam Kumar Mandal, HOD (R&D Department), IKOP Sdn. Bhd., Kulliyyah
of Pharmacy, International Islamic University Malaysia (IIUM), Jalan Sultan
Ahmad Shah, Bandar Indera Mahkota, 25200, Kuantan, Pahang Darul Makmu r, Malaysia.
E-mail: mandalju2007@gmail.com,
Mobile: +60 109062750, Fax: +60 9571 6775.
Abstract
This research work deals with formulation development and
characterization of an analgesic pediatric ointment. The ointment was
developed with menthol and camphor as active ingredients at lower
concentrations than used for an adult formulation. The formulation was
novel as it replaced the oleaginous hydrocarbon petroleum jelly base of
conventional ointment by olive oil, a more acceptable excipient to the
pediatric patients. The developed formulation was compared against
iKOOL, a commercial analgesic ointment with similar composition
produced by IKOP Sdn. Bhd., IIUM, Malaysia with respect to various
quality control tests. The developed formulation showed encouraging
results,but needs further investigation to convert it into a commercial
product.
Key words:
Analgesic ointment, Menthol, Camphor, Formulation development.
Running title:
Analgesic ointment formulation.
INTRODUCTION
As compared to oral administration, topical administration of analgesic
medicine provides some distinct and important advantages. It overcomes
gastric irritation and undesirable effects on cognition and level of
consciousness associated with its oral administration [1]. Topical
administration ensures a more even and continuous application of the dosage
for a long period directly to the site of the pain. Topical ointment
containing menthol and camphor has been used safely for many decades for
analgesic relief of minor aches and pains of muscles and joints [2-4].
Additionally, they are indicated for general relief and suppression of
common cold symptoms as cough, sore throat, nasal congestion together with
Their natural origin has helped them to be used commercially as safe and
efficacious as compared to their synthetic counterparts. Menthol (C 10H20O; molecular weight, 156; a terpene alcohol) is
obtained either from naturally occurring peppermint oil of synthesized by
hydrogenation of thymol. In lower concentrations (1% or less), menthol
depresses cutaneous sensory receptors, while at higher concentrations
(1.25% to 16%), it stimulates sensory receptors and thus it gives its
counter-irritant action [5]. Camphor (a terpenoid class of compound) is
obtained from the wood of Cinnamomum Camphora L. [2]. The
analgesic and counter irritant effect of camphor is quite established,
however its pharmacological mechanism is not yet fully understood [6]. In
one study, Moqrich et al. [7] found that camphor activates TRPV3, a member
of the transient receptor channel that causes excitation and
desensitization of sensory nerves. When menthol and camphor are used
together as analgesic, they provide synergistic action [8]. In few reports,
menthol was found to cause allergic contact dermatitis [9,10] and systemic
allergic reactions [11]. Camphor occurs in in two enantiomerform; natural
one is dextrorotatory (D-camphor), while the synthetic one form
laevorotatory (L-camphor). As compared to the natural one, synthetic
camphor is more toxic orally as claimed by toxicity study in mice [12].
There are many branded products, both as over-the-counter (OTC) and
prescription, in the form of ointment, liniment, and cream available in the
markets containing the active ingredients like menthol and camphor.
However, these commercial products contain high amount of active drugs (5
to 10% menthol and 5% camphor) as well as excipients. As a result, these
products are not safe when applied to the pediatric population. Topical
therapies, especially, analgesic and counter irritant medications that are
widely used for pediatric patients are often misused. Their unique type of
skin which is quite immature with respect to barrier property is more
susceptible to permeation of drugs as well as excipients as compared to the
adult skin; this may often lead to undesirable toxicity [13]. Skin surface
area to body weight ratio in infants and children is significantly higher
than the adults, which pose a great risk of accumulation of drugs in their
body. US Food and Drug Administration (FDA) does not entertain the use of
commonly prescribed topical medicines to pediatric patients as around 70%
of them are devoid of any pediatric labelling [13].
At this juncture, the objective of the present work was to explore the
feasibility of formulation and characterization of an ointment containing a
reduced amount of menthol (2% w/w) and camphor (2% w/w) for pediatric
patients. An attempt was made to reduce the amount mineral oil as ointment
base and replaced it with live oil. This was fundamentally proposed for
safety and patient compliance reasons, as olive oil is being historically
used to soothe baby skin rashes in addition to its well known skin
moisturizer effect. A analgesic ointment formulation, iKOOL produced by
IKOP Sdn Bhd., IIUM, Malaysia was used as reference formulation to compare
with the developed formulation.
MATERIALS & METHODS
Materials
Olive oil, menthol crystal, camphor, eucalyptus oil, and peppermint oil
were obtained as generous gift from IKOP Sdn., IIUM, Malayisa. For GC
analysis, camphor (≥95.50 %), and menthol (≥98.20 %) (Sigma-Aldrich, USA),
and petroleum benzine (boiling point 80 - 100 ⁰C) (Fischer scientific Co.,
UK) were generously donated by IKOP Sdn Bhd., Malaysia. Analgesic ointment
iKOOL used as reference sample was also obtained from IKOP Sdn. Bhd., IIUM,
Malaysia. Instrumental facility to prepare the ointment and characterize
like water bath, rotational viscometer, centrifuge, stability chamber, gas
chromatography etc were also provided by IKOP Sdn. Bhd.
Manufacturing Method
Composition of the ointment formulation is shown in Table 1. Water bath
(WNB 8 Memmert GmbH +Co. KG) was adjusted to 750 C. Beeswax and
olive oil were transferred in a glass beaker placed on that water bath.
Beeswax was allowed to melt and mixed with olive oil completely. Then
camphor was added to the mixture with consistent stirring. The mixture was
cooled down by lowering the bath temperature until 600C.
Immediately, menthol, eucalyptus oil, and peppermint oil were added to the
mixture and allowed to cool down to 280C until it forms an
ointment with the required consistency.
Table 1: C
omposition of the analgesic baby ointment.
|
Material name
|
Quantity (g/100g)
|
|
Olive oil
|
60 to 73%
|
|
Bees wax
|
17 to 30%
|
|
Menthol crystal
|
2
|
|
Camphor
|
2
|
|
Eucalyptus oil
|
3
|
|
Peppermint oil
|
3
|
|
Total
|
100gm
|
Optimization of the Ointment Base
In order to optimize the viscosity of the formulation, few experimental
trials were carried out with different ratios of beeswax and olive oil as
shown in Table 2. iKOOL ointment formulation prepared by IKOP Sdn. Bhd was
taken as the reference product to optimize the developed formulation.
Table 2:
Various experimental ratios of beeswax and olive oil for analgesic baby
ointment formulation.
|
Number of trials
|
Ointment base
|
|
Beeswax % (w/w)
|
Olive oil % (w/w)
|
|
1
|
30
|
60
|
|
2
|
25
|
65
|
|
3
|
20
|
70
|
|
4
|
15
|
75
|
|
5
|
17
|
73
|
Characterization of the Developed Ointment
Viscosity Measurement
The viscosity of the developed formulation was determined by Brookfield
Rotational Viscometer (DV-II+PRO Digital viscometer). A 200 ml test sample
was taken in a clean and dry 500 ml beaker and the viscosity of the test
sample was determined by using spindle no 6 at speeds of 20,50,100 and 150
r.p.m. During the measurement, the spindle was lowered upto a point where
the spindle does not hit the bottom of the beaker. The temperature was kept
uniform for all the test samples.
Centrifugation Stressed Test
The stability of the optimized formulated and the reference ointment
formulation was evaluated using the forced centrifugation test. This test
was carried out as per the method described by Baie & Sheikh [14]. Both
the formulations were subjected to speed ranged from (2000 to 14000) rpm
for 15 minutes in a centrifuge apparatus (Heraeus Megafuge 8 Centrifuge,
Fisher Thermo Scientific, Model No. FB15067) and phase separation of the
formulation, if any, was evaluated by visual observation.
Microbial Contamination Test
Developed ointment formulation was tested for both TAMC (total aerobic
microbial count) and TYMC (total combined yeast/mould count). An amount of
10 g of the sample was suspended in sodium chloride peptone solution (PH
7). One (1) ml of the prepared previous solution was added to a sterile
petri dish. Casein Soya bean digestive agar medium and sabouraud dextrose
agar were prepared for cultivation of bacteria and fungi respectively, by
pouring them into the prepared petri dishes. Then the petri dishes were
incubated at 300-350 for bacteria and 200
-250 for fungi for five days. One (1) ml of the buffered sodium
chloride was used as a negative control.
After the incubation period, colonies were counted using the manual colony
counter. Not more than 104 for bacteria and not more than 10 2 for fungi per gm /ml was considered as the limit of the
microbial contamination test .
Assay for the Developed Formulation for Menthol and Mamphor by Gas
Chromatography
Both the test and reference formulations were tested for the content of
menthol and camphor by gas chromatography method. According to USP
pharmacopeia, the formulation should contain not less than 90.0 percent and
not more than 110.0 percent of the labeled amounts. A Gas Chromatography
(GC) system (Agilent Technologies, 7890B GC System) The test was performed
according to the following procedure:
Preparation of Solutions:
Diluent: Petroleum benzene
Blank: 1ml of the diluent was transferred into GC vial after filtration
through 0.45 µm filter paper.
Standard stock solution of camphor and menthol: 1 g of camphor was weighted
and transferred into a 100ml volumetric flask, containing 60ml of diluent,
and after sonication takes place, the volume topped up with the diluent.
The same method was adopted for menthol stock preparation using 1g of the
menthol material.
Working Standard solution: 1 ml of both camphor and
menthol standard stock solution was transferred into 10ml volumetric flask
containing 5 ml diluent, mixed well and the volume was topped up, and
finally transferred into the GC vials after passing them through a 0.45 µm
membrane filter paper
Sample stock solution: 1 g of the ointment sample was transferred into
100ml volumetric flask containing 60ml diluent, then sonicated to ensure
sample dissolution, and make up the volume with diluent.
Working sample solution: 1 ml of the sample stock solution
is transferred into 10ml volumetric flask containing 5 ml diluent, mixed
well and the volume was topped up. Finally, they were transferred into GC
vial bypassing a µm 45.0 filter paper.
Chromatographic parameters:
GC detector: Flame ionization detector (FID)
Gas carrier: Helium
Column: VF-WAXms
Length: 30.0 m
Internal diameter: 250.00 µm
Film thickness: 0.25 µm
Mode: Split (Ratio 20:1)
Injection volume: 1µL
Run time: 14 Minute
The content of active ingredient was calculated based on the following
equation:
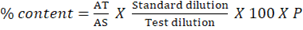 …………..equation 1
…………..equation 1
Where, AT: Area of peak response due to analyte content in sample solution
AS: Area of peak response due to analyte content in standard solution
WT: Weight of sample taken
P: % of purity of analyte standard
Stability Study
An accelerated stability study was carried out according to the ICH
guidelines at 400C/75% RH. Due to time constraint, only one
sample of three months was analysed. The formulation was filled in the
aluminum collapsible tubes; physical appearance, viscosity, and drug assay
were analysed as evaluation parameters.
RESULTS AND DISCUSSION
Several experimental trials were carried out to investigate the best ratio
of olive oil and beeswax for the required viscosity of the formulation. The
results are shown in Table 3 and Figure 1 to Figure 4 based on the highest
obtained torque values. It was found that the ratio of 17:73 w/w for
beeswax and olive oil was just enough to provide the required viscosity
like the reference product. The formulation with this composition of
beeswax and olive oil (17:73 w/w) was selected as the optimized
formulation. This selection was based on in its consistency and viscosity
resemblance to the commercial iKOOL formulation prepared by IKOP Sdn. Bhd.,
IIUM, Malaysia. The higher the concentration of beeswax, the higher was the
viscosity. The optimized formulation resulted comparable characterization
parameters in comparison with the commercial product. Table 4 shows the
results of centrifugation stressed test subjected to the optimized test and
reference formulation. As such, both the formulations resulted similar type
of physical stability profile; they withstand physical separation by
centrifugation force exerted at 6000 RPM for 15 minutes, initiate
separation at 8000 RPM, but completely separate at 10000 RPM. No bacteria
or fungi growth was revealed after microbial contamination test performed
on the optimized formulation. 3 months of accelerated stability study at
400C and 75% RH for three months showed good stability profile for the
optimized formulation. The formulation was physical stable without any kind
of phase separation. As evident from the GC chromatograms (Figure 5 and
Figure 6), menthol and champhor were well separated without any kind of
interference. Assay results for both active pharmaceutical ingredients
after 3 months of accelerated stability study is shown in Table 5. It
indicates that both camphor (2.12 %) and menthol (1.96 %) were within the
accepted range (±10% of the label claim) of their content percentage (2%
w/w).
Table 3:
Viscosity measurement results based on the highest obtained torque value.
|
Number
|
Ratio (bees wax /Olive oil)
|
Torque value / Speed
|
Viscosity value (cP)
|
|
1
|
(25:65)
|
93.6 / 20 r.p.m
|
46800.00
|
|
2
|
(20:70)
|
92.3 / 150 r.p.m
|
6153.33
|
|
3
|
(17:73)
|
53.9 / 150 r.p.m
|
3593.33
|
|
4
|
(15:75)
|
61.7 / 200 r.p.m
|
3085.00
|
|
5
|
iKOOL
|
65.6 / 50 r.p.m
|
13120.00
|
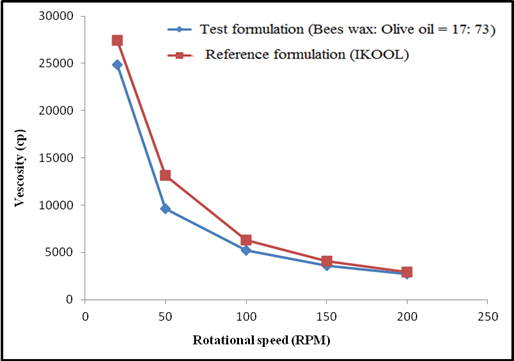
Figure 1:
Viscosity of test formulation (beeswax and olive oil ratio-17:73) and
reference formulation (iKOOL).
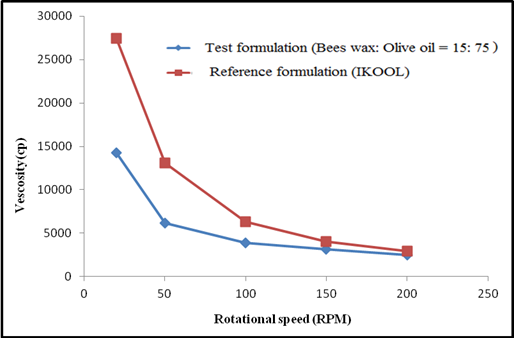
Figure 2:
Viscosity of test formulation (beeswax and olive oil ratio-15:75) and
reference formulation (iKOOL).
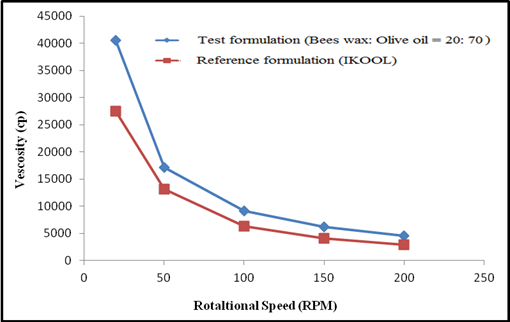
Figure 3:
Viscosity of test formulation (beeswax and olive oil ratio-20:70) and
reference formulation (iKOOL).
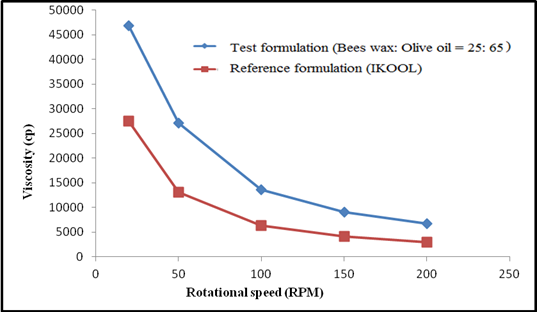
Figure 4:
Viscosity of test ointment (beeswax and olive oil ratio-25:65) and
reference product (Original iKOOL)
Table 4:
Results of centrifugation stressed test subjected to optimized test and
reference formulation.
|
Centrifugation speed
|
Observation
|
|
Optimized test formulation
|
Reference formulation
|
|
2000
|
No separation
|
No separation
|
|
4000
|
No separation
|
No separation
|
|
6000
|
No separation
|
No separation
|
|
8000
|
Slight separation
|
Slight separation
|
|
10000
|
separation
|
Separation
|
|
12000
|
separation
|
Separation
|
|
14000
|
separation
|
Separation
|
Table 5:
Assay results for menthol and camphor analytical results in mix standard
and optimized test formulation after 3 months of accelerated stability
study.
|
Sample identity
|
Retention Time
(minutes)
|
Area
|
Height
|
Width
|
Area
|
Symmetry
factor
|
|
Menthol in mixed standard
|
2.756
|
620.5
|
773.2
|
0.0124
|
47.821
|
1.132
|
|
Menthol in test sample
|
2.757
|
642.9
|
791.1
|
0.0135
|
46.748
|
1.112
|
|
Camphor in mixed standard
|
3.213
|
677.1
|
788.7
|
0.013
|
52.179
|
1.192
|
|
Camphor in test sample
|
3.214
|
732.3
|
835.2
|
0.0142
|
53.252
|
1.193
|
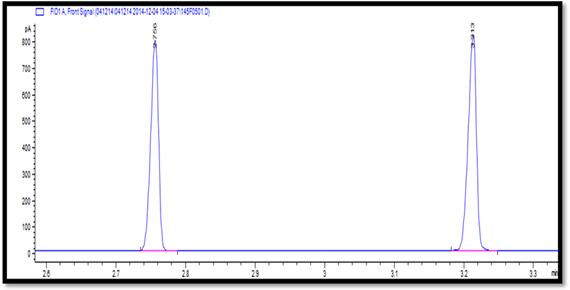
Figure 5:
Representative GC chromatograms showing the separation of menthol (2.758
minutes) and camphor (3.213 minutes) in mix standard.
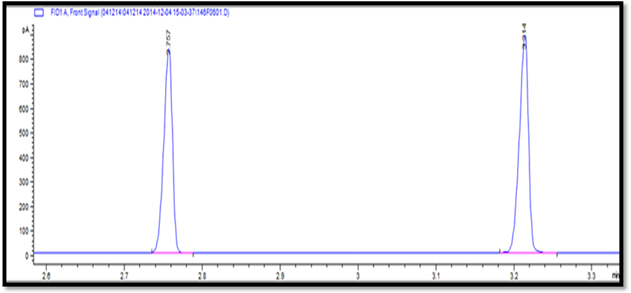
Figure 6:
Representative GC chromatograms showing the separation of menthol (2.757
minutes) and camphor (3.214 minutes) in test sample subjected to 3 months
accelerated stability study.
CONCLUSION
An optimized analgesic ointment formulation with a composition of 2%
menthol and 2% camphor together with beeswax and olive oil mixture (17: 73
w/w) as a base was developed for pediatric patients. The various evaluation
parameters carried out to assess the quality of the
developed pediatric ointment formulation and they showed satisfactory
results when compared with the reference iKOOL ointment prepared by IKOP
Sdn. Bhd, IIUM, Malaysia. The viscosity of the optimized formulation was
also satisfactory when compared with the reference product. Moreover, the
assay results for both the active ingredients after a period of 3 months
accelerated stability study was within the accepted range for the active
pharmaceutical ingredients. The developed analgesic pediatric ointment
containing menthol and camphor may be further investigated to commercialize
in future as a replacement of the conventional analgesic ointments
available in the market.
REFERENCES
[1] H.K. Archer, M.S. Pettit, Analgesic and antiphlogistic compositions and
therapeutic wrap for topical delivery, U.S. Patent No. 5, 976, 547.
Washington, DC: U.S. Patent and Trademark Office, 1999.
[2] P. Zuccarini, Camphor: risks and benefits of a widely used natural
product, J. Appl. Sci. Environ. Manag. 3 (2009) 69-74.
[3] C. Gaudioso, J. Hao, M.F. Martin-Eauclaire, M. Gabriac, P. Delmas,
Menthol pain relief through cumulative inactivation of voltage-gated sodium
channels, Pain 153 (2012) 473-484.
[4] P. Johar, V. Grover, R. Topp, D.G. Behm, A comparison of topical
menthol to ice on pain evoked tetanic and voluntary force during delayed
onset muscle soreness, Int. J. Sports Phys Ther. 7 (2012) 314-322.
[5] T. Patel, Y. Ishiuji, G. Yosipovitch, Menthol: a refreshing look at
this ancient compound, J. Am. Acad. Dermatol. 57 (2007) 873-878.
[6] H. Xu, N.T. Blair, D.E. Clapham, Camphor activates and strongly
desensitizes the transient receptor potential vanilloid subtype 1 channel
in a vanilloid-independent mechanism, J. Neurosci. 25 (2005) 8924-8937.
[7] A. Moqrich, S.W. Hwang, T.J. Earley, M.J. Petrus, A.N. Murray, K.S.
Spencer, M. Andahazy, G.M. Story, A. Patapoutian, Impaired thermosensation
in mice lacking TRPV3, a heat and camphor sensor in the skin, Science 307
(2005) 1468-1472.
[8] T.M. Wang, L.Q. Ding, H.J. Jin, R. Shi, J.S. Wu, L. Zhu, Y.Q. Jia, Y.M.
Ma, Simultaneous quantification of multiple volatile active components in
rat plasma using a headspace-solid phase dynamic extraction method coupled
to gas chromatography-tandem mass spectroscopy: application in a
pharmacokinetic study of Longhu Rendan pills, RSC Adv. 5 (2015)
29631-29638.
[9] A. Aguirre, J.M. Oleaga, R. Zabala, R. Izu, J.L. Díaz‐Pérez, Allergic
contact dermatitis from Reflex® spray, Contact Derm. 30 (1994)
52-53.
[10] S.M. Wilkinson, M.H. Beck, Allergic contact dermatitis from menthol in
peppermint, Contact Derm. 30 (1994) 42-43.
[11] E.M. McGowan, Menthol urticarial, Arch. Dermatol. 94 (1966) 62–63.
[12] N. Chatterjie, G.J. Alexander, Anticonvulsant properties of
spirohydantoins derived from optical isomers of camphor, Neurochem. Res. 11
(1986)1669-1676.
[13] D.W. Metry, A.A. Hebert, Topical therapies and medications in the
pediatric patient, Pediatr. Clin. North Am. 47 (2000) 867-876.
[14] S. H. Baie, K.A. Sheikh, The wound healing properties of Channa
striatus-cetrimide cream-wound contraction and glycosaminoglycan
measurement, J. Ethnopharmacol. 73 (2000) 15-30.